On the question of 65. This ester does not undergo Claisen condensation because it has no a-hydrogens and cannot undergo enolization.

Solved Draw The Product Of The Below Intramolecular Claisen Chegg Com
I already draw the product to the reaction but Im not sure if I did it right.

. Draw only the major transformation of the starting material. 1-Write the complete stepwise mechanism for this reactionShow all electron flow with arrows and draw all intermediate structures. An intramolecular aldol condensation c.
An intramolecular reaction is one in which the. Intramolecular Aldol Condensation Reaction. Draw the major product of this Claisen condensation reaction.
CH3CH2ONa CH3CH2OH Ph H3C H3. Draw the major product of this Claisen bartleby. Ester and enolate ion.
Now the mind the base usually abstract. If an ester does not undergo Claisen condensation explain why it does not. Draw the product of the below intramolecular claisen condensation Valentines Day is approaching it is just per month absent but There are tons of stuff to arrange from dresses to the consuming area from bouquets to your items baskets Now we have to arrange anything for our family members.
An intramolecular Claisen condensation b. Intramolecular Aldol condensations happen when a single molecule contains 2 reaction aldehydeketone groups. David Rawn in Organic Chemistry Second Edition 2018 Claisen Condensation of Thioesters.
Acetoacetic ester can be prepared by the Claisen self-condensation reaction of ethyl acetate. Solution for Draw the major product of the Claisen condensation reaction between two molecules of this ester. Draw The Product Of The Below Intramolecular Claisen Condensation.
The reaction Opare Im behavior it No Alba hide isnt for example Benzali aid that a sitting and hydrated in business So sodium acetate in mild base. The mechanism of the Dieckmann condensation is the same as a. An enol Exhibit 23-3 Consider the data below to answer the following.
OH O Ph-CH2 CH2C-C-C-OCH3 CH2Ph 9. Here is the way how I got the product in my question. Determine what dicarbonyl compound is needed to synthesize each compound below by an intramolecular aldol condensation reaction.
A Robinson annulation d. The product β-keto ester product of the Claisen condensation is more acidic than the reactants. Draw the structure of the product you would expect to obtain by Claisen condensation of each of the following esters.
Also a reverse Claisen condensation occurs in the catabolism of. The product off the working condensation is to be form. In this tutorial video we examine the intramolecular Claisen condensation known as the Dieckmann cyclization.
An -unsaturated ketone c. My task was to do an intramolecular Claisen condensation with this molecule. This video shows you how to identify the correct alpha carbon and carbonyl will.
This brought on on me that would release that is generated civilized the carbon you. An -unsaturated aldehyde d. The product of this reaction is.
2-Ethyl acetate can be prepared from ethanol as the only. Be sure to use the Ph short-cut for the benzene ring. The aldol condensation of ketones with aryl aldehydes to form αβ-unsaturated derivatives is called the Claisen-Schmidt reaction.
A Claisen condensation between two different esters is called a crossed Claisen condensationThere are two main types of crossed Claisen that we will go over in this post. A variation on the Claisen condensation is an important biochemical reaction responsible for carboncarbon bond formation in the biosynthesis of fatty acids. No person couldnt take any more.
Learn this topic by watching Intramolecular Condensation Retrosynthesis Concept Videos. Show the mechanism for each reaction. Draw the structure of the product that is expected from intramolecular aldol condensation of each of the following compound and show the mechanism for each conversion.
One is the reaction between esters both having alpha hydrogens and the other is when only one of the partners has alpha hydrogens. If both esters have ɑ hydrogens the reaction is not synthetically. Draw the structures of the esters that would undergo Claisen condensation to give the following -keto ester.
Give the structures of the ester precursors for the following Claisen condensation product and formulate the reaction. Draw structural formulas for the reactants. Refer to Exhibit 23-2.
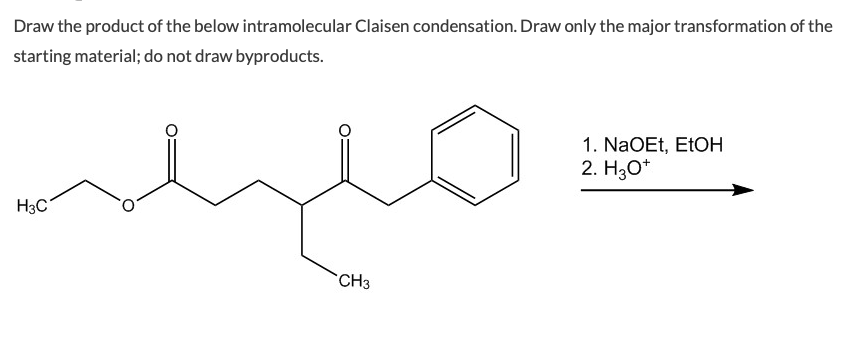
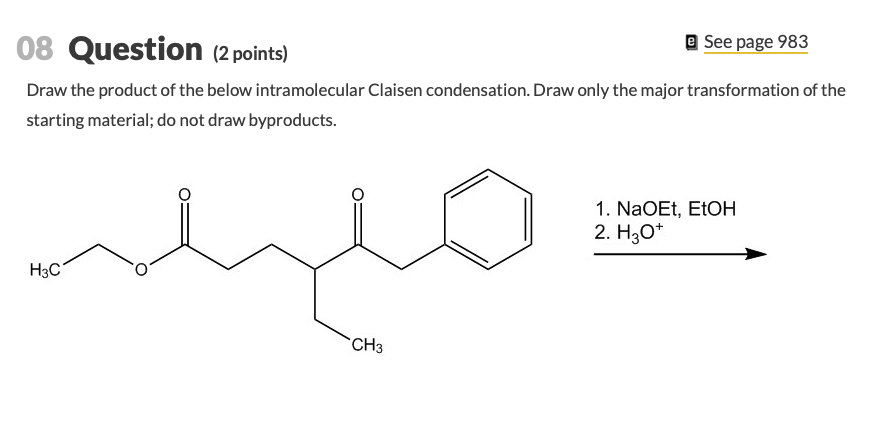
The reaction proceeds when a strong base is present and the product of the reaction is a beta-keto ester or a beta-diketone. Diesters can undergo an intramolecular reaction called the Dieckmann condensation to produce cyclic beta-keto esters. Draw the product of the below intramolecular Claisen condensation.
A -unsaturated aldehyde b. One full equivalent of base must be used in the Claisen condensation. An intramolecular Claisen condensation.
If a compound does not undergo aldol self-condensation explain why it does not. Because its a ketone to our aldehydes and youre going to get a cyclic enone. There should be only one product since there is symmetry.
When a diester self-condensates the resulting product is called a cyclic β-ketoester. 239 Mixed Claisen Condensation Strategies are similar to that of the mixed aldol reaction. This relies on the fact that the addition of an enolate to a ketone is not particularly favorable whereas the addition of an enolate to an aldehyde is favorable.
Draw the product of the below intramolecular Claisen condensation. Could anyone help me out or confirm the solution. Deprotonation of the product drives the reaction forward.
In the next video Im going to show you what happens for intramolecular condensations of esters. Do not draw side products. This is known as a Dieckmann Condensation or Intramolecular Claisen.
This means that the aldehyde is consumed while the ketone serves to provide the. A Michael reaction 7. Draw the structure of the aldol self-condensation product for each of the following compounds.
VIII- Consider the reaction below to answer the following questions. This reaction works best with 16-diesters which produce five-membered rings and 17-diesters which produce six membered rings. The compound shown below is the product of a Claisen condensation.
Draw the structures of the products formed when the following diesters undergo intramolecular Claisen condensation Dieckmann Condensation. When the alpha carbon of one group attacks the other the molecule attacks itself forming a ring structure. C O CO Et H 3 C H H C 2CEt.
The Claisen condensation reaction is an organic coupling reaction that results in the formation of a C-C bond between either a single ester and one carbonyl compound or between two esters.

For The Following Reaction Draw The Major Organic Products Study Com
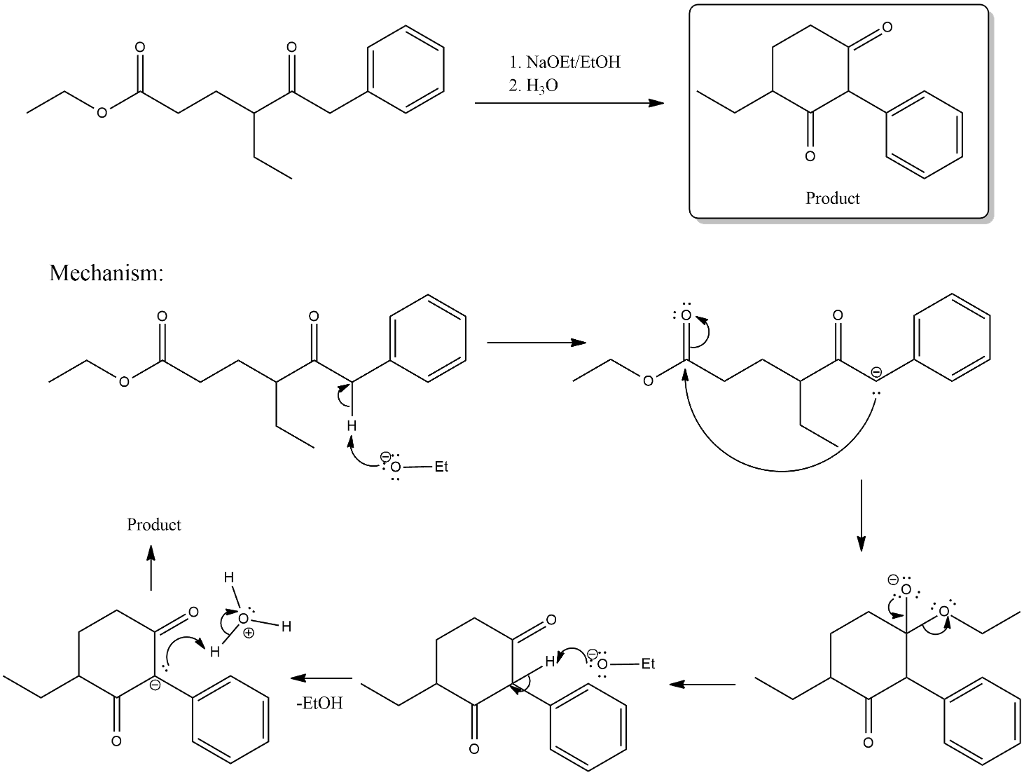
Oneclass Draw The Product Of The Below Intramolecular Claisen Condensation Draw Only The Major Tran
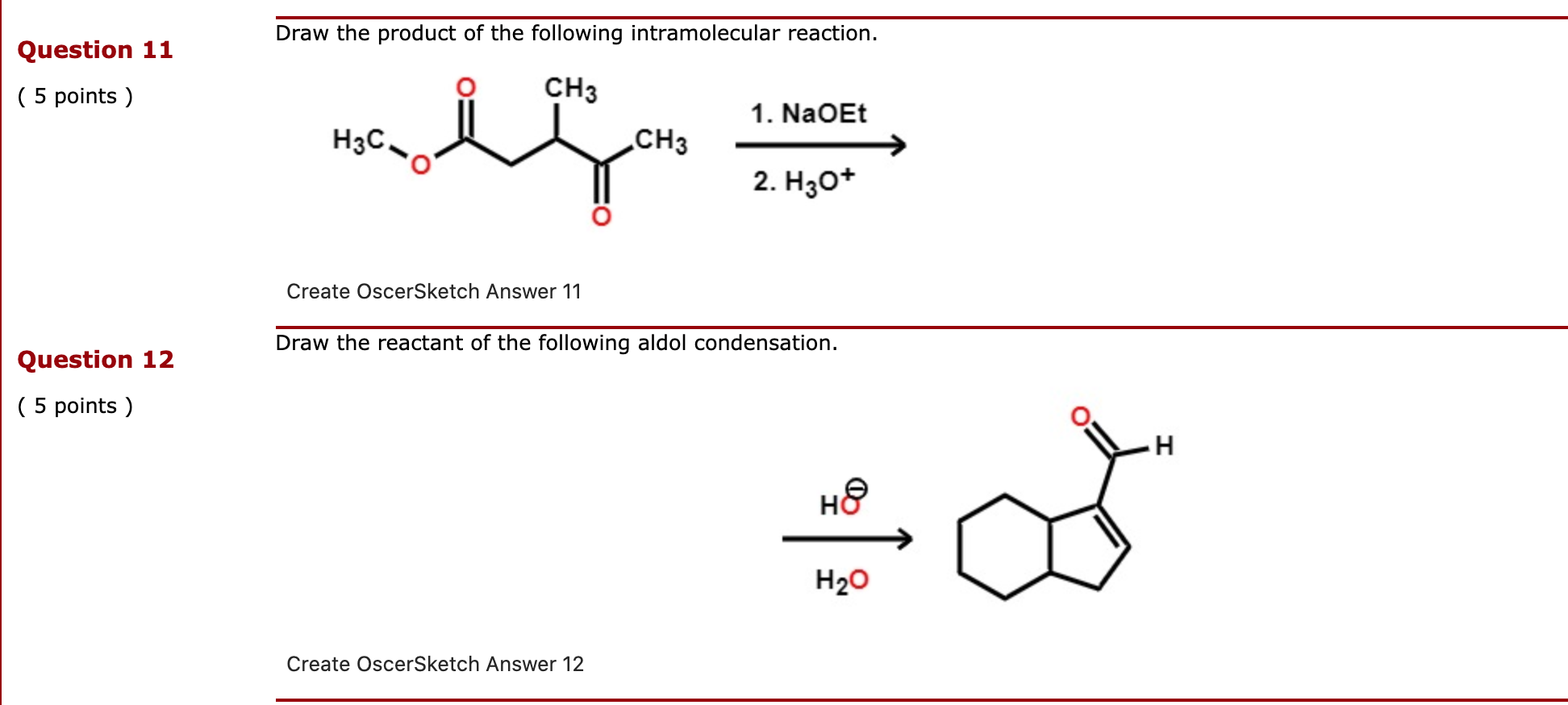
Solved Draw The Product Of The Following Intramolecular Chegg Com
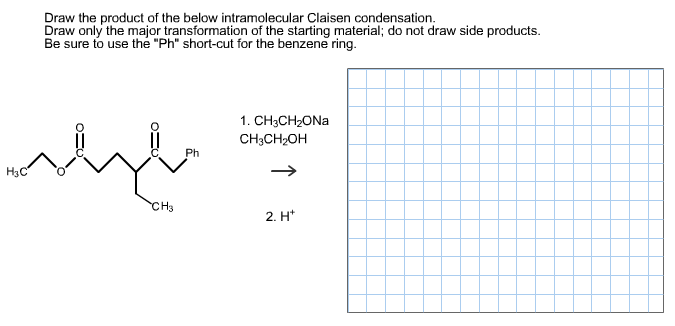
Solved Draw The Product Of The Below Intramolecular Claisen Chegg Com

Solved Draw The Product Of The Below Intramolecular Claisen Chegg Com
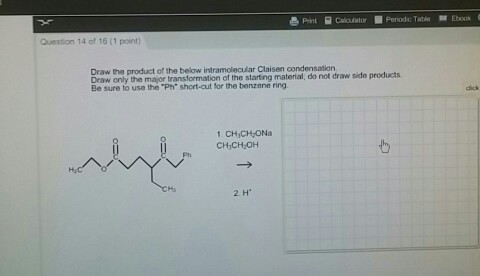
Solved Draw The Product Of The Below Intramolecular Claisen Chegg Com
Solved Draw The Product Of The Below Intramolecular Claisen Chegg Com
Solved 08 Question 2 Points E See Page 983 Draw The Chegg Com
0 comments
Post a Comment